- Preface
- Acknowledgements
- Chapter 1: Welding Overview
- Chapter 2: Safety
- Chapter 3: Terms, Joints, & Edge Preparation
- Chapter 4: Tools & Welding Tables
- Chapter 5: Shielded Metal Arc Welding
- Chapter 6: Wire Feed Welding
- Chapter 7: Gas Tungsten Arc Welding
- Chapter 8: Oxyacetylene
- Chapter 9: Controlling Distortion
- Chapter 10: Cutting Processes
- Chapter 11: Brazing & Soldering
- Chapter 12: Common Problems & Solutions
- Chapter 13: Design Tips
- Chapter 14: Fabrication & Repair Tips
- Chapter 15: Tools & Tooling
- Chapter 16: Pipe & Tubing
- Chapter 17: Metallurgy
- Chapter 18: Power Supplies & Electrical Safety
- Chapter 19: Bending & Straightening
- Index
- Credits
Chapter 8
Oxyacetylene
Facts do not cease to exist because they are ignored.
—Aldus Huxley
Introduction
One of the earliest welding processes, oxyacetylene welding was first used industrially in the early years of the twentieth century. Although this process makes excellent welds in steel, today it is not a major industrial welding process except with makers of light aircraft and race car frames, since now there are other more efficient welding processes available. However—and this is a big however—oxyacetylene has many other important uses: cutting, hardening, tempering, bending, forming, preheating, post-heating, brazing, and braze welding. Because of the precise control the welder has over its heat input and its high-temperature flame—together with its low equipment cost, portability and versatility—oxyacetylene torches remain an essential tool. No welding shop or even industrial shop is complete without one.
This chapter is devoted to oxyacetylene equipment because of its key importance to welders and machinists. It pays special attention to safety because, as with all effective tools, oxyacetylene is inherently hazardous. With this equipment, accidents happen amazingly fast, and once they begin—they cannot be stopped.
We will cover the theory and use of oxyacetylene in depth so you can use the equipment with confidence and safety. This chapter will prepare you for the later chapters on cutting, brazing, and bending because many components and techniques are common to all of these processes. There are also several commercial issues on cylinder ownership which this chapter will explain.
Oxyacetylene offers several advantages over other torch fuels:
- Heats metal quickly. Only welding arc plasma, nuclear explosions, and the sun produce higher temperatures than a 5600°F oxyacetylene flame.
- Provides excellent control of heat input and puddle viscosity as well as good control of bead size and shape.
- The single, low-cost outfit is multifunctional. It not only welds, it cuts, heats, bends, anneals, and brazes.
Section I – Equipment
Welding Outfit
Figure 8-1 shows the components of a complete oxyacetylene welding outfit: an oxygen cylinder, an acetylene cylinder, pressure regulators, hoses and a torch. Later in the chapter each part will be discussed in depth. Oxyacetylene equipment is light enough to be readily portable and it requires no external electrical power source.
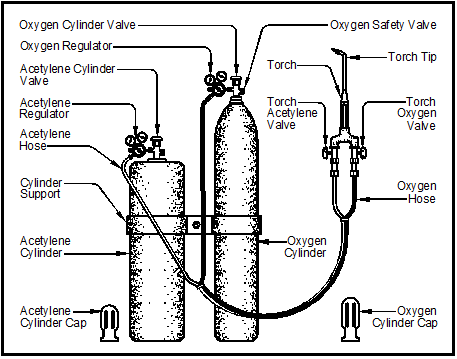
Section II – Gases
Acetylene
Acetylene is a clear, lighter than air gas with a chemical formula of C2H2. It is man-made and does not occur in nature. Each molecule of this hydrocarbon compound contains two carbon atoms and two hydrogen atoms. Placing calcium carbide in water releases acetylene gas. One pound of calcium carbide generates about 10 ft3 of acetylene gas. Pure acetylene has an ether-like odor, but commercial quality acetylene, the type used by welders, has a garlic-like odor from the impurities found in the calcium carbide.
The chemical equation of combustion of acetylene in a neutral flame is:
C2H2 + 2.5 O2 ® 2 CO2 + H2O
One part acetylene and two and a half parts oxygen combine to produce a neutral flame. Equal volumes of acetylene and oxygen from the compressed gas cylinders combine with an additional one and a half parts of oxygen, which comes from the surrounding atmosphere. This type of flame has just the right amount of fuel and oxygen so that after combustion there is neither an excess of oxygen nor of fuel.
Acetylene is potentially dangerous for two reasons:
- Acetylene forms explosive mixtures with air at concentrations from 2.5–80 percent. This is the widest explosive range of any common gas and almost insures an explosion when leaking acetylene ignites.
- Acetylene gas must never be drawn from its cylinder at pressures above 15 psi because it can spontaneously and explosively disassociate into its components. This is why acetylene regulator gauges display a red warning arc above 15 psi.
Oxygen
Atmospheric air is repeatedly cooled and compressed until it becomes a very cold liquid. As this liquid air is gradually warmed, each of its component gases reaches its vaporization temperature, comes out of the liquid air, and separates into its individual gases, one of which is oxygen. This process is called the fractional distillation of liquid air. Other gases important in welding—nitrogen, carbon dioxide and argon—are also made using this process. Oxygen can also be made by electrolysis of water, but for industrial quantities this is not cost-effective due to the expense of the electricity needed.
Other Welding Fuels
Though not strictly used for oxyacetylene, this is a good time to discuss other welding fuels. For economy and safety reasons, other gases, such as natural gas, propane, hydrogen and proprietary gases based on mixtures of these, are frequently used for non-welding processes. Table 8-1 shows the maximum temperature achievable with these alternative fuel gases as compared with the temperature of acetylene.
These other fuels work well for soldering, brazing, preheating and oxygen cutting, but are seldom used for welding. Slightly different torch tips may be needed if alternative fuel gases are used with an oxyacetylene outfit. Where even lower temperatures are acceptable, such as when sweating copper tubing or performing small soldering tasks, a single cylinder of fuel gas using only atmospheric oxygen is effective and economical. In some foreign countries where acetylene is not readily available, gasoline is used, but only for cutting.
|
Fuel Gas |
Oxygen-to-Fuel Gas Combustion Ratio |
Neutral Flame Temperature with Oxygen ºF ºC |
Total |
Specific (Air = 1.0) |
|
|
Acetylene |
2.5 |
5589 |
3087 |
1470 |
0.906 |
|
Propane |
5.0 |
4579 |
2526 |
2498 |
1.52 |
|
Propylene |
4.5 |
5250 |
2900 |
2400 |
1.48 |
|
Natural Gas |
2.0 |
4600 |
2538 |
1000 |
0.60 |
|
Hydrogen |
0.5 |
4820 |
2660 |
325 |
0.07 |
Table 8-1. Combustion properties of fuel gases.
MAPP Gas
MAPP gas is a trademarked name owned by Pétromont & Co. of Varennes, Quebec, Canada, for a fuel gas based on a mixture of methylacetylene-propadiene propane. Although this gas has not been made in North America since 2008, much of it is still around. Its many advantages make it likely to eventually reappear on the market. The Chinese manufacture an identical product, but it is not yet available in the U.S.
MAPP gas was developed as a lower-cost substitute for acetylene in air-MAPP torches that are used for plumbing and refrigeration soldering and in oxygen-MAPP torches used for cutting, brazing, and bending. MAPP gas is not suitable for welding because its high hydrogen content causes embrittlement in steel welds.
Here are some of its advantages:
- MAPP gas is safer to handle than acetylene because it does not decompose explosively at pressures above 15 psi.
- MAPP can be stored and transported in cylinders without the inert filler and acetone that acetylene requires, so more gas can be delivered in a given size cylinder. Also, unlike acetylene, MAPP cylinders can be filled and drained rapidly, rather than the seven hours required to fill and empty an acetylene cylinder.
- When MAPP gas is used with oxygen its temperature is slightly lower than acetylene-oxygen, but the heat is more evenly distributed throughout the flame envelope, making it excellent for brazing, heating, and bending.
Today, propylene is offered as a substitute for MAPP gas. It works acceptably well in air-propylene torches, but not very well in oxygen-propylene torches. MAP/Pro, one of the commercial names for the MAPP substitute, propylene, is confusingly similar to MAPP. Although the 14.1 ounce cylinders are the same yellow color and carry the same UPC code as the discontinued MAPP gas cylinders, the two are actually different products sold in deceptively similar packaging.

